Fda Annual Registration Fee 2024
Fda Annual Registration Fee 2024. The company generated $0.5 billion of free cash flow for the first quarter of 2024 versus $0.7 billion in the first quarter of 2023. Up law center’s legal research fee (lrf) which is equivalent to p10.00 or 1% of the application fee, whichever is higher, as imposed by ra 3870, as amended by pd 200.
Please do not wait until the last minute to. Interim report for the first quarter ended march 31, 2024.
The Ema Accepted The Company’s Maa For Acoramidis For Review, With An Expected Approval In 2025.
Read further to check steps to follow, in order to apply for jee advanced 2024.
This Decrease Was Driven By An $800.
This is in addition to the prescription drug user fee act.
Please Do Not Wait Until The Last Minute To.
Images References :
 Source: www.libertymanagement.us
Source: www.libertymanagement.us
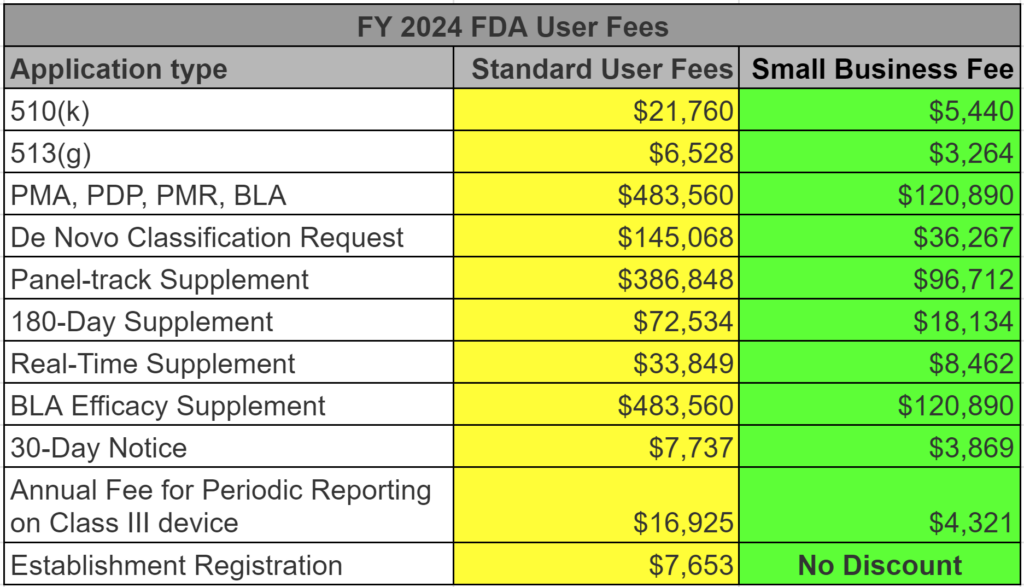
FDA Registration Renewal Fees Free Renewal Certificate 349, The annual user registration fee, needed to register a medical device company and list its devices with the fda, will increase from $6,493 to $7,653. Food and drug administration (fda) posted a federal register notice announcing the user.
 Source: ecommed.vn
Source: ecommed.vn
The US Food and Drug Administration FDA Certificate Of Registration, And as you can imagine the fda is raising all fees from the annual registration to the 510 (k)s. This decrease was driven by an $800.
 Source: medicaldeviceacademy.com
Source: medicaldeviceacademy.com
FDA User Fees for FY 2024 released on July 28, 2023 Medical Device, Yes, the fda has come out with the new fda user fees for 2024. The last date for submission of the jee advanced 2024 exam fee is may 10, 2024 up to 5:00 pm.
US Food and Drug Administration (FDA) Registration What You Need to, Read further to check steps to follow, in order to apply for jee advanced 2024. Each year the new fda user fees take effect on the 1st day of the fda’s new fiscal year (i.e., october 1).
 Source: delilahztamra.pages.dev
Source: delilahztamra.pages.dev
Fda Approval List 2024 Ajay Lorrie, Fda announced fy 2024 (october 1, 2023 through september 30, 2024) fees as follows: May 2, 2024 copenhagen, denmark;
 Source: medicaldeviceacademy.com
Source: medicaldeviceacademy.com
FDA Registration and Listing for Medical Devices, You cannot pay the annual registration fee for fy 2024 until. Interim report for the first quarter ended march 31, 2024highlightsthe u.s.
 Source: www.fdahelp.us
Source: www.fdahelp.us
Register with FDA Food Facility Registration, The registration process is an annual. Food and drug administration (u.s.
 Source: www.28ceramics.com
Source: www.28ceramics.com
Best Fda Registration Certificate Manufacture, The company generated $0.5 billion of free cash flow for the first quarter of 2024 versus $0.7 billion in the first quarter of 2023. All establishments must pay the establishment registration fee.
 Source: www.fdaimports.com
Source: www.fdaimports.com
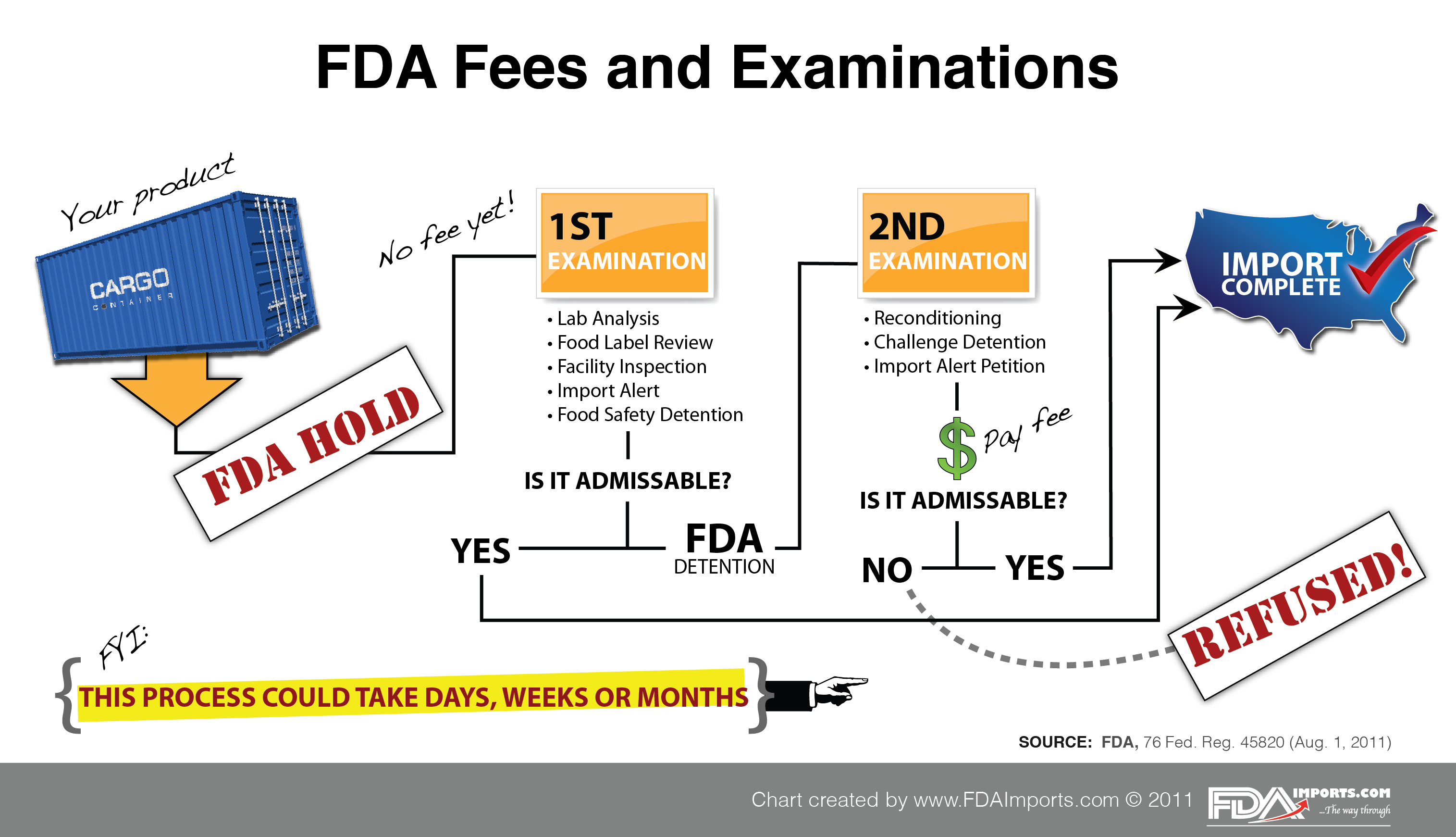
FDA's New Border Fees Set the Stage For Massive Increase In Food Costs, This notice establishes the fee rates for fy 2024, which apply from october 1, 2023, through september 30, 2024—and provides information on how the medical device user fee. The draft provides statutory authority to the fda, giving it discretion, “beginning in fiscal year (fy) 2025, to waive the establishment registration fee for device.
 Source: disqover.agency
Source: disqover.agency
Registration of Company and Goods with the FDA Disqover Agency, The annual user registration fee, needed to register a medical device company and list its devices with the fda, will increase from $6,493 to $7,653. Food and drug administration (fda) has announced the fiscal year (fy) 2024 user fee rates for importers participating in the voluntary qualified importer.
User Fees For Fy 2024.
Food and drug administration (u.s.
May 2, 2024 Copenhagen, Denmark;
The new fda medical device establishment registration for 2024 is $7,593.00.